SERVICES
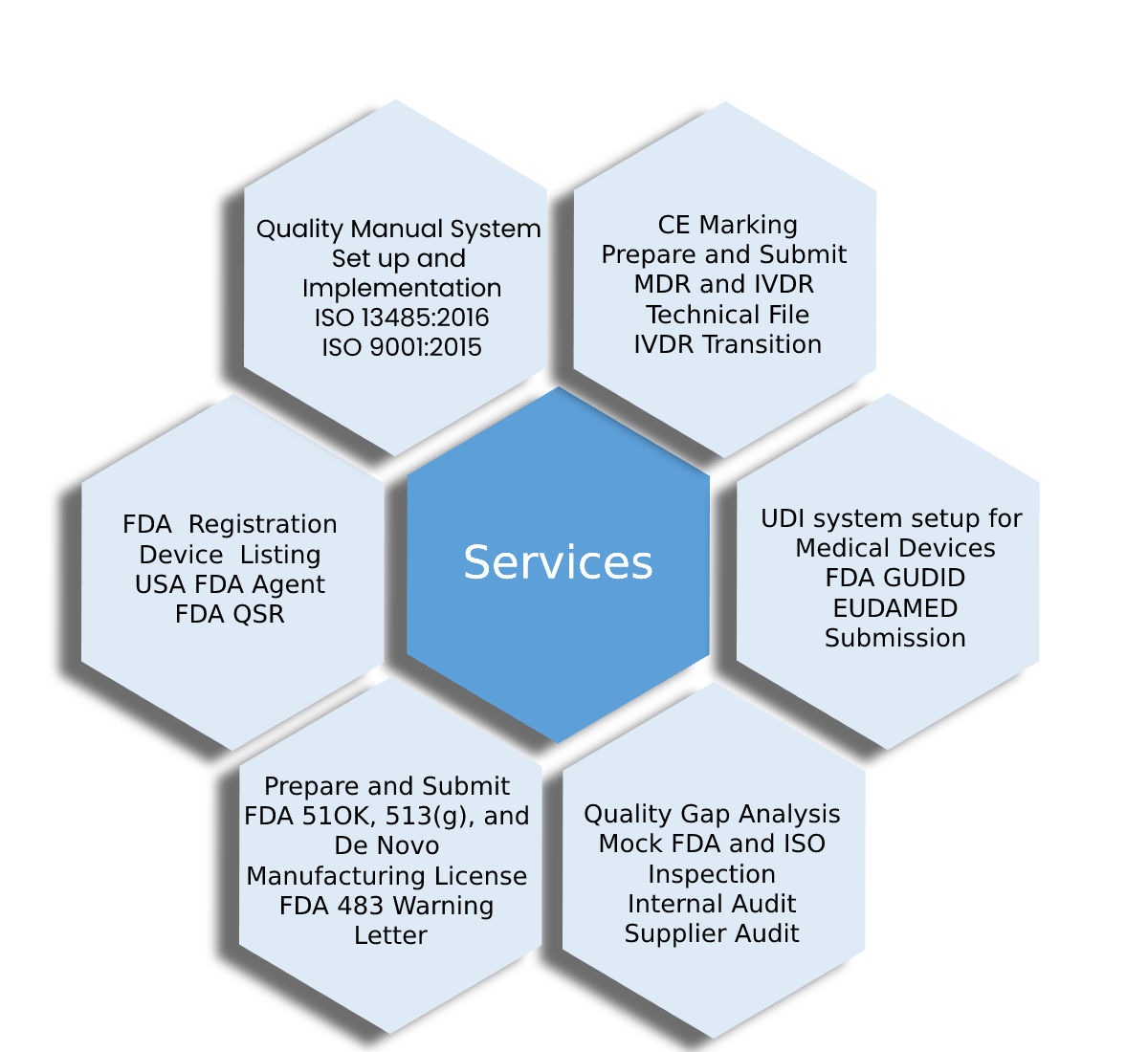
Helping You
Conform To Various Regulatory Guidelines
With Regulatory Solutions, Inc. your company can always remain compliant with the medical device manufacturing standards and regulations set by regulatory authorities. With our more than 25 years of experience in the field, we can be trusted to provide full-service, reliable consulting and support to biotech companies all over the world.
Let Us Handle Your Regulatory Compliance Needs
With our extensive expertise, you can count on us to handle any concern with utmost excellence and dedication. For more information, feel free to get in touch with us today.

